Rocket fuels are the lifeblood of space exploration, serving as the primary means of propelling spacecraft beyond Earth’s atmosphere.
The choice of fuel is critical, not just for achieving the necessary thrust to escape Earth’s gravitational pull, but also for ensuring the sustainability and efficiency of space missions.
Rocket fuels vary widely in composition, each with unique properties that make them suited to different types of missions and rockets.
That is why I feel disclosing this subject is important for understanding the crucial concepts.
Types of Rocket Fuels
First, let us address the types of rocket fuels.
Bipropellant Rockets
Bipropellant rockets are a type of rocket engine that utilize two separate propellants, typically in liquid form, to create a hot gas for propulsion.
These engines are distinct from single-propellant solid rockets and hybrid rockets, which combine a solid propellant with an injected fluid.
Liquid Hydrogen and Liquid Oxygen (LH2/LOX)
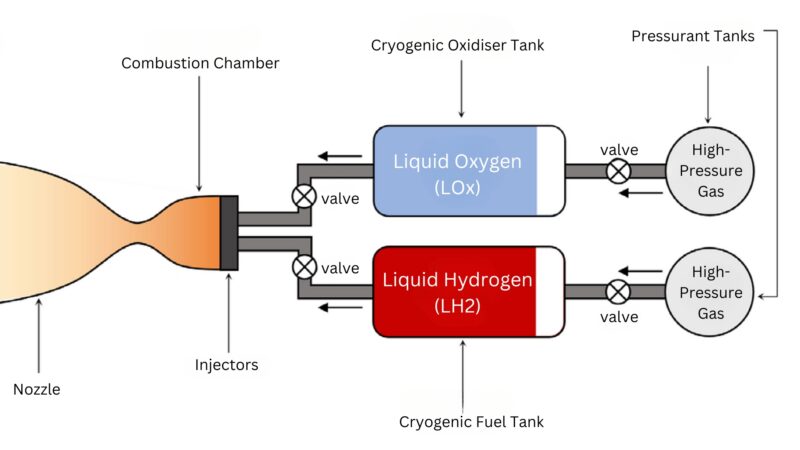
Liquid hydrogen (LH2) combined with liquid oxygen (LOX) offers the highest efficiency among current rocket fuels.
Its high performance is attributed to the high specific impulse it provides.
The extremely low temperatures required to keep hydrogen in liquid form present significant storage and handling challenges.
The complex infrastructure needed to maintain these conditions can increase the cost and complexity of space missions.
Kerosene and Liquid Oxygen (RP-1/LOX)
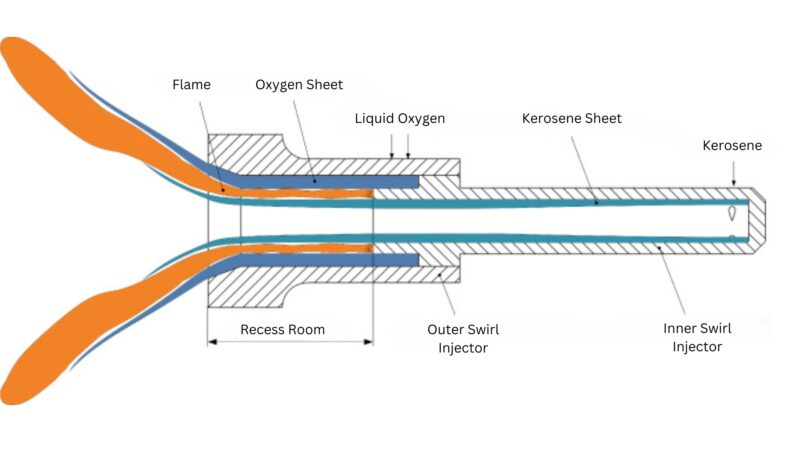
Kerosene, when used with liquid oxygen as RP-1/LOX, is a staple in rocket propulsion, commonly used in many first-stage rocket engines including those of the Falcon 9.
It is less efficient than LH2/LOX but offers advantages in terms of density and storability at room temperature, making it less complex to handle and store.
This makes RP-1/LOX a cost-effective option for a wide range of missions.
Methane and Oxygen (Methalox)
Methane is a relatively new entrant in the field of rocket propellants, used in combination with oxygen to form Methalox.
It is seen as a promising fuel for future Mars missions due to its potential for in-situ resource utilization—Mars’ atmosphere could theoretically be processed to extract methane, facilitating longer missions or even permanent settlements.
Monopropellants
A monopropellant rocket, also known as a monochemical rocket, is a type of rocket that utilizes a single chemical compound as its propellant.
These rockets are frequently used for small-scale applications such as attitude and trajectory control in satellites, rocket upper stages, manned spacecraft, and spaceplanes.
Hydrazine
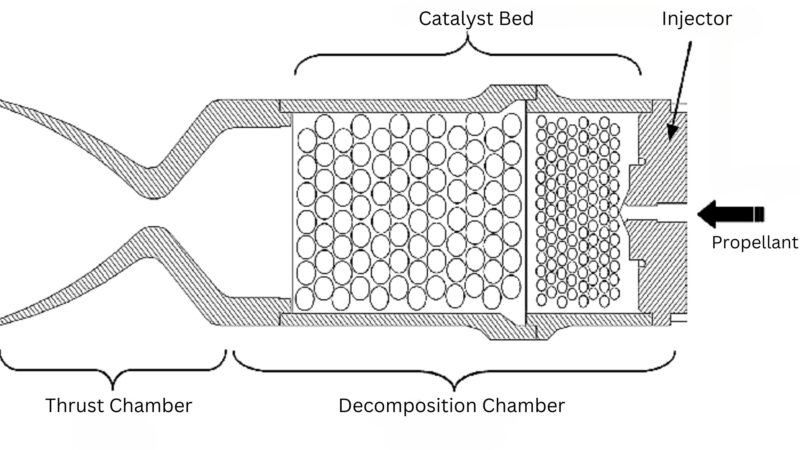
Hydrazine is widely used in satellite propulsion for orbit adjustments and attitude control.
As a monopropellant, it decomposes exothermically upon contact with a catalyst, releasing gas that provides thrust without the need for a separate oxidizer.
Its ease of storage and handling makes it advantageous for in-space maneuvers, although its toxicity poses significant hazards during ground handling.
Hypergolic Propellants
Hypergolic propellants consist of rocket fuel mixtures that ignite automatically when their two components—typically a fuel and an oxidizer—come into contact.
These propellants are prized for their ability to remain liquid at room temperature, allowing for engines that can be ignited reliably and multiple times without the need for external ignition sources.
UDMH and N2O4
Unsymmetrical dimethylhydrazine (UDMH) combined with nitrogen tetroxide (N2O4) forms a hypergolic propellant mixture that ignites spontaneously in contact with each other.
These propellants are favored for their reliability and the simplicity of engine design, as they do not require an ignition system.
Their high toxicity and corrosive nature raise serious safety and environmental concerns.
Evolution of Rocket Fuels

The evolution of rocket fuels has been influenced by a myriad of factors including technological advancements, environmental concerns, and geopolitical contexts.
Initially, rocket fuels were selected based on performance criteria alone, leading to the widespread use of highly toxic substances like hydrazine.
Over time, the environmental impact and safety issues associated with toxic propellants have driven research toward greener alternatives.
Geopolitical factors have also played a role, as countries seek to develop independent capabilities in space technology and minimize reliance on foreign resources.
Challenges and Trade-offs
The design and selection of rocket fuels involve a complex balance of factors including performance, safety, cost, and environmental impact.
Storage issues present a significant challenge, especially for cryogenic fuels like LH2 that require extremely low temperatures.
Toxicity is another major concern, particularly with hypergolic fuels, which are hazardous to handle and pose significant health risks.
These challenges impact not only the design of the rocket engines but also influence the broader mission architecture, dictating what is feasible in terms of range, payload capacity, and mission duration.
Innovations and Future Directions
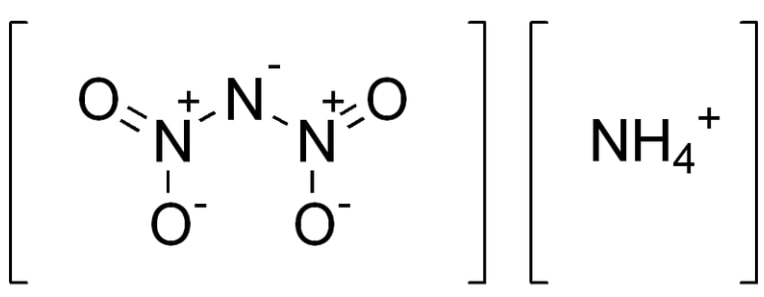
In the quest for safer and more efficient rocket propulsion, significant advancements are being made in the development of non-toxic, high-performance fuels.
Ammonium dinitramide (ADN) is one example of a green propellant that offers high performance without the toxicity of traditional hypergolic fuels.
Researchers are also exploring the potential of solid-state and hybrid propellants that could offer the best of both worlds—safety and efficiency.
The ongoing innovation in rocket fuel technology promises to expand the possibilities of future space exploration, reducing the environmental footprint of rocket launches and enhancing the safety of space travel.
Related Posts:
- New Discoveries on the Dark Side of the Moon - What we Know?
- The Meaning of Max Q in Rocket Science
- 9 Major Bodies of Water in Florida - Discovering its…
- The 10 Most Famous Astronauts in The World ─ From…
- Answers About Universe Expansion, the Vacuum in…
- Voyager 2 Communication Update and What Occured After?









